Types of Spectra Continuous Emission and Absorption
Characteristics of Continuous, Emission, and Absorption Spectra
Part A
There are three general types of spectra: continuous, emission, and absorption. Each is characterized by a different distribution of the wavelengths (i.e., colors) of radiation. Sort the images of the three types of spectra into the appropriate bins.

Part B
The universe is filled with objects of extreme temperatures and densities, both high and low. The differences among the three types of spectra result from the various physical conditions in which the light is emitted, or through which it travels, before it is observed on Earth. The following three diagrams illustrate the use of a simple spectroscope in a laboratory. Match each diagram to the appropriate spectra. Note that a light bulb can be thought of as a hot, dense source of radiation.

Part C
No astronomical object that produces a continuous visible spectrum of light has ever been observed. However, there are many astronomical objects that produce emission or absorption spectra. Read the following descriptions of astronomical objects, and then sort the labeled images into the appropriate bins according to the type of spectrum each object produces.
- Emission nebula: a cloud of hot, interstellar gas glowing as a result of one or more nearby young stars that ionize the gas.
- Planetary nebula: a glowing cloud of hot, low-density gas that is ejected from a red-giant star.
- Sun: a glowing ball of extremely dense gas powered by nuclear fusion in its core, but surrounded by a low-density, cooler atmosphere.
- Atmosphere on Titan: a layer of cool, low-density gas confined close to the surface of Titan, one of Saturn's moons.

Components and Structure of the Atom
Part A
The atom consists of three types of subatomic particles: protons, neutrons, and electrons. The electron is by far the lightest of the three, while the much heavier proton and neutron have masses very similar to each other. Two of the types of particles carry an electrical charge, while the third is neutral. Label the subatomic particles and appropriate charges by their relative locations.

Part B
Of the three types of subatomic particles, only neutrons do not carry charge. Protons carry a positive charge, and electrons carry a negative charge. Protons and neutrons are bound in the nucleus, while electrons orbit the nucleus. When the number of each type of subatomic particle in an atom changes, the characteristics defining the atom also change. Match the appropriate phrases with the type of subatomic particle that completes the defining characteristic.

Part C
In the classical view of the atom, Bohr pictured electrons orbiting the positively charged nucleus similar to how the planets orbit the Sun. While this picture was not entirely correct, it provides a good framework in which to make calculations about the energies of electrons. Different from the predictions of Newtonian mechanics, which allows any energy to be possible, Bohr described the electron orbits (now called orbitals) as having specific energies. Rank the following electron energy states according to their electron energies.

Part D
The Bohr model accounted for most of the general characteristics of the atom. However, the modern model based on quantum mechanics explains that, although the energy of each orbital is fixed, the orbital radius is actually an average distance. The result is a "cloud" where the electron would most probably be located.
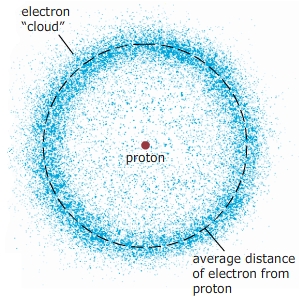
When electrons are excited to different energy levels, the average radii from the nucleus also changes. Rank the following electron energy states according to the average distance of the electron from the nucleus. Rank from largest to smallest distances.

Electromagnetic Radiation
Part A
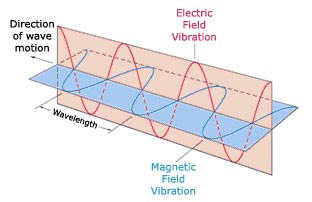
Light is a wave, and, like all waves, it is characterized by specific physical characteristics. Identify the key physical characteristics of a wave in the figures below. Figure A shows a wave as a function of time, and Figure B shows a wave as a function of space.

Part B
If you throw a rock into a pond, it creates a wave in the water. What is responsible for creating an electromagnetic wave?
A vibrating charged particle
Part C
The law of physics state that a magnetic field must accompany a changing electric field, and a change in one must create a change in the other. Together, electric and magnetic fields make up electromagnetic waves, which carry energy and information from one part of the universe to another. Electromagnetic waves share many properties with ordinary waves, but they also have a number of unique characteristics. Using the figure below and your knowledge from Parts A and B, complete the following statements about the specific properties of electromagnetic radiation.

Ranking Task: Reflecting Telescopes and Light Collection
Part A
Listed following are the names and mirror diameters for six of the world's greatest reflecting telescopes used to gather visible light. Rank the telescopes from left to right based on their light-collecting area from largest to smallest. For telescopes with more than one mirror, rank based on the combined light-collecting area of the mirrors.

Part B
Shown following are the primary mirror arrangements and total light-collecting area of five different telescopes. Each mirror uses a different segmented arrangement, but assume that they are all equivalent in quality and in their ability to focus light. Also assume that the telescopes use identical detectors and have the same observing conditions. Rank the telescopes from left to right based on their ability to detect very dim objects, from greatest to least. To rank two (or more) telescopes as equal, drag one on top of the other(s).

Part C
Shown following are the primary mirror arrangements and total light-collecting area of five different telescopes. Notice that although the arrangements look similar to those in Part B, the light-collecting areas are not the same. Also listed is an amount of time (exposure time) that each telescope will be pointed at the same distant galaxy. Again assume that the quality of these mirrors, the detectors, and the observing conditions are identical. Rank the telescopes from left to right based on the brightness of the image each telescope will take of the galaxy in the time indicated, from brightest to dimmest. To rank two (or more) telescopes as equal, drag one on top of the other(s).

Sorting Task: Characteristics of Reflecting and Refracting Telescopes
Part A
Listed following are distinguishing characteristics and examples of reflecting and refracting telescopes. Match these to the appropriate category.

Visual Activity: Atmospheric Absorption of Light at Different Wavelengths
Part A
Which of the following forms of light can be observed with telescopes at sea level?
visible light
radio waves
Part B
If our eyes were sensitive only to X rays, the world would appear __________.
dark because X-ray light does not reach Earth's surface
Part C
If you had only one telescope and wanted to take both visible-light and ultraviolet pictures of stars, where should you locate your telescope?
in space
Sorting Task: Interaction of Light and Matter
Part A
Listed following are various physical situations that describe how light interacts with matter. Match these to the appropriate category.

Sorting Task: Temperature Scales
Part A
Each of the following items states a temperature, but does not tell you whether the temperature is measured on the Fahrenheit, Celsius, or Kelvin scale. Match the items to the appropriate temperature scale.

Source: https://orange520.blogspot.com/2015/09/masteringastronomy-assignment-0.html
0 Response to "Types of Spectra Continuous Emission and Absorption"
Postar um comentário